Sodium hypochlorite is the salt of the hypochlorous acid, which is the easiest form of an oxygen acid of chlorine. Solutions of sodium hypochlorite are commonly also known as bleach. Seamlessly, disinfection and bleaching are the common applications of solutions of sodium hypochlorite.
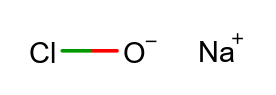
The most important facts
- Sodium hypochlorite is an acid and the easiest form of a chlorine oxygen acid.
- The substance is the salt of the hypochlorous acid.
- The salt of the stable form has a pure white appearance.
- Solutions are also commonly known as bleach.
- Sodium hypochlorite solutions are often used for cleaning, disinfecting and bleaching applications.
- Concentration is an important factor when using this chemical.
- The higher the concentration the riskier the application.
- Hardware stores, drugstores and online market places sell this chemical in low concentrations.
- Different application bottles are available.
- Used wrong, the chemical substance causes strong irritations up to caustic or even toxic damaging.
Safety advise for a secure handling
While using sodium hypochlorite, this chemical should be handled at all times safely. Before using this product, make yourself familiar with the potential hazards and follow the precautionary statements at all times. Sodium hypochlorite is a strong oxidizing agent and could potentially release hydrogen or chlorine gases in contact with other chemicals.
There is a risk of fire and unexpected explosion when sodium hypochlorite reacts unintentionally with other chemicals. For example, reducing agents, amines, organic substances, and acids (e.g. hydrochloric acid, nitric acid) are to be mentioned here. Those reactions can release chlorine gas, other chlorine compounds, or nitrous gases, which can seriously attack your lungs and membranes. Skin or eye contact of sodium hypochlorite causes irritations in very low concentrations, but in higher concentrations, the substance reacts even caustic. Especially in private households, the accidental mixing of acidic cleaners with bleach can easily occur and cause critical damage to personal life.
Besides these above-mentioned reactions, warming or direct sunlight can lead to the decomposition of sodium hypochlorite with releasing of different chlorine gas component combinations. During the decomposition, those gases can cause pressure to build up until the packaging bursts.
GHS hazard statements:
H314, H318, H400, H410
GHS precautionary statements:
P260, P264, P273, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P391, P405, and P501
GHS 5 Corrosion 
GHS 9 Environment
Synonyms and Identifiers
| IUPAC | Sodium hypochlorite |
| CAS Number | 7681-52-9 |
| UN number | 1791 |
| InChI | InChI=1S/ClO.Na/c1-2;/q-1;+1 |
| SMILES | [Na+].[O-]Cl |
| PubChem CID | 23665760 |
| Synonyms | Antiformin Bleach Chloride of soda |
Applications for Sodium hypochlorite
Disinfection for household
Sodium hypochlorite is an essential active ingredient in some household cleaners. For example, mold remover or some pipe cleaner contain this chemical substance to strengthen their cleaning capabilities. Sometimes, you can find the advertisement for “active chlorine” on the outside. However, from a chemical point of view, hypochlorite is the active part, but pure chlorine should not be released according to the safety statements above. Therefore, read the safety instructions carefully. The concentrations in household cleaners can differ and might be between 5-7 %.
Disinfection for swimming pools
This chemical substance is also a potential solution to clean your private swimming pool. Taking sodium hypochlorite for disinfection for swimming pools into account, the concentration of the pure chemical is often higher than in common cleaning agents, like those mentioned above. This is because the water in the pool would again reduce the concentration of the chemical. However, a too low concentration would limit the disinfection of the pool or even show no effect. By using sodium hypochlorite as a disinfectant for your swimming pool, follow the instructions on the packaging to calculate the ideal concentration or volume to use for your pool and handle the chemical safely.